At the end of 2020, the National Health Surveillance Agency (ANVISA) published four guidelines on its prior consent procedure, which all pharmaceutical patent applications in Brazil must undergo before the Brazilian Patent and Trademark Office (BRPTO) conducts the technical examination. Since 2001, when this requirement was included in the IP Law, ANVISA and the BRPTO's relationship has been conflicted, especially since ANVISA has had the right to reject patent applications based on patentability requirements for more than 15 years. Most applicants are strongly against ANVISA's interference because the technical examination of patent applications should not be made by a regulatory agency. Moreover, such double analysis significantly delays technical examinations, thereby increasing the BRPTO's backlog. Fortunately, in 2017 ANVISA's controversial interference in the technical examination of patent applications appeared to have finally been settled by means of an agreement between the agency and the BRPTO.
At present, ANVISA analyses the following.
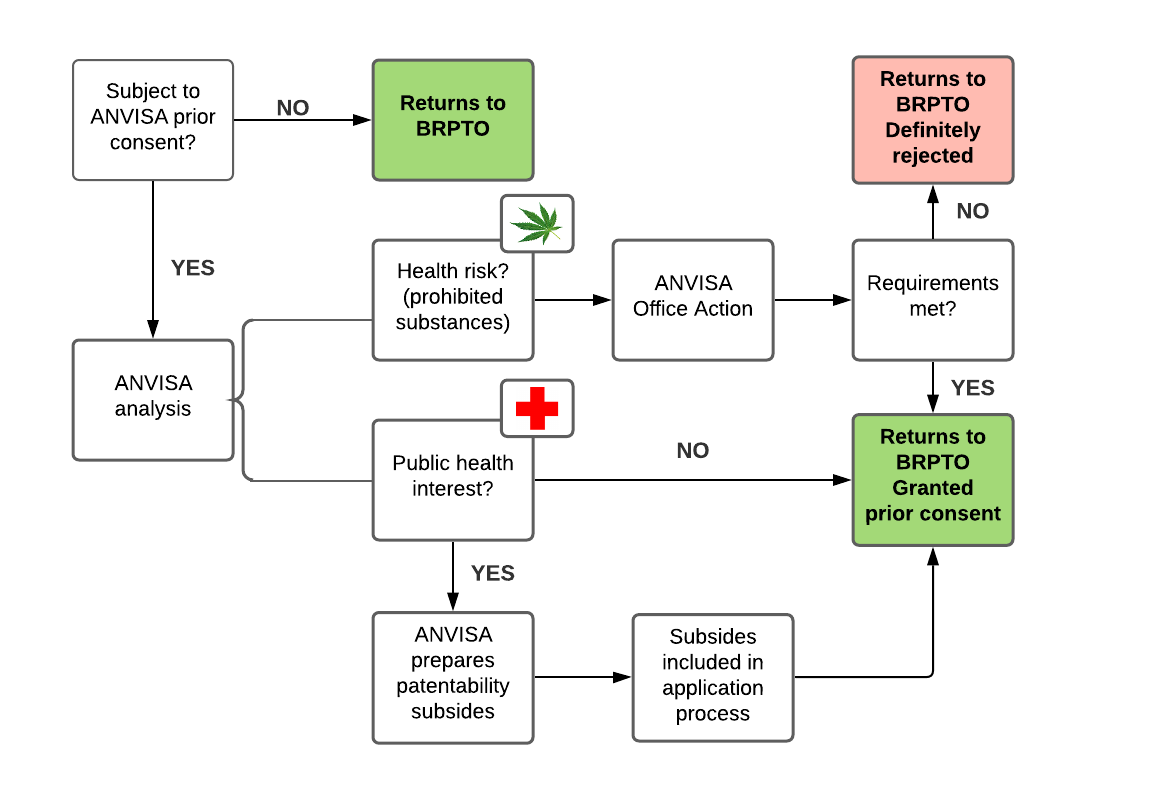
Figure 1: Analysis carried out by ANVISA
As Figure 1 demonstrates, the only scenario in which an application leaves ANVISA definitely rejected is when the application claims protection for substances prohibited in Brazil or plants that contain intoxicating or psychotropic compounds. However, even in such cases, an office action is issued and the applicant can amend the application or submit arguments in an attempt to receive ANVISA's prior consent. Examples of said substances and plants include:
- methadone;
- morphine;
- oxycodone;
- opium;
- cannabis sativa;
- erythroxylum coca; and
- lophophora williamsii.
ANVISA can still issue an opinion on the patentability requirements; however, since the agreement entered into force, ANVISA's opinion is considered a third-party observation. Therefore, BRPTO examiners need not follow ANVISA's opinion.
Although at first glance the publication of ANVISA's guidelines could be seen as a deviation from the 2017 agreement, the guidelines only put into words ANVISA's internal procedure, with no intention to bypass the agreement made with the BRPTO. This is corroborated by the initial excerpt of all four guides, which states that:
- the guides are non-normative regulatory instruments, which aim to better define the procedure; and
- the prior consent procedure follows the agreement made with the BRPTO.
The first guide summarises ANVISA's prior consent procedure while the second and third guides analyse health risks and public health interest, respectively. The fourth guide assembles and explains the patentability requirements used by ANVISA examiners. This document gathers information from the IP Law and the BRPTO's guidelines and includes some of ANVISA's points of view that differ from the BRPTO's current position.
The third and fourth guides focus on public health interest – the fourth guide being of particular relevance. However, before providing an analysis on this specific guide, it is necessary to take one step back and explain what kind of substances are considered to be of a public health interest in Brazil. Patent applications for pharmaceutical products or processes for human use in connection with a wide range of diseases are considered under ANVISA's patentability analysis from a public health standpoint, including with regard to:
- cancer;
- viral diseases;
- neglected diseases;
- degenerative diseases;
- mental disorders;
- some immunosuppressants;
- products obtained from biological routes;
- vaccines;
- serums; and
- hemoderivatives.
The fourth guide highlights the patentability requirements and the BRPTO's peculiarities that ANVISA examiners – who need not be knowledgeable on this matter – need to know. In this regard, the guide covers not only the IP Law but also the BRPTO's guidelines and resolutions currently in force. Of particular note is a summary of aspects wherein ANVISA has a different and more limited interpretation of the IP Law than the BRPTO. Selection patents, second medical use, hybridomas and antibodies are examples of aspects where the BRPTO and ANVISA diverge.
Considering ANVISA's position, a stricter interpretation of the IP Law is likely. However, the divergence in specific topics should not be a concern for pharmaceutical patent applicants that wish to file patent applications in Brazil since ANVISA's opinion is considered in technical examinations only if the BRPTO examiner concludes that ANVISA's position is based on the BRPTO's examination guidelines. Consequently, the final decision on the patentability of pharmaceutical patent applications is made exclusively by the BRPTO.





