There has been some concern regarding the statistics periodically issued by the Patent Trial and Appeal Board (PTAB) of the US Patent and Trademark Office, owing to the fact that the reported numbers overlook multiple inter partes review challenges to the same patents and, potentially, different outcomes in those challenges. Inter partes reviews filed on drug patents (ie, patents that are listed in the Food and Drug Administration's (FDA) Orange Book and patents that have been identified in proceedings as reading on FDA Purple Book-listed biologic drugs) were monitored; this update reveals that while certain drug patents have been challenged in multiple inter partes review petitions, concern as to different outcomes – at least in the final written decisions that have been issued to date – appears to be unfounded. These decisions have been consistent: either all instituted claims have been held unpatentable or all instituted claims have been held patentable. The biggest news is that drug patents challenged in multiple inter partes reviews – at least those that reach final written decisions – have a much greater chance of being found unpatentable than drug patents that have been challenged in only one inter partes review. The percentage is 63% for duplicative final written decisions, compared to 44% for non-duplicative decisions.
As of July 20 2017 there have been more than 350 inter partes review petitions filed against Orange Book patents, and more than 70 inter partes review petitions filed against biologic drug patents. Of these drug patent inter partes review petitions:
- 67% have been resolved (institution has been denied in 23%, a final written decision has been issued in 26% and inter partes review has otherwise been terminated – eg, following settlement – in 18%);
- 16% have been instituted and trial is pending; and
- the remaining 17% are awaiting an institution decision (see figure 1).
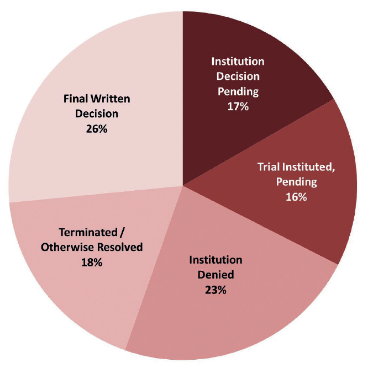
Figure 1: status of drug patent inter partes reviews as of July 20 2017
Duplicative final written decisions
This update focuses on the subset of drug patent inter partes reviews that have been resolved, and in particular on those final written decisions concerning drug patents that were also the subject of at least one other final written decision ('duplicative final written decisions'). As of July 20 2017, of the more than 420 inter partes review petitions filed to date against drug patents, there have been at least 72 duplicative final written decisions. Of these, 55 (76%) were on joined inter partes reviews.(1) The remaining 17 final written decisions were separate decisions issued on inter partes reviews that had not been joined. Of the joined inter partes reviews that reached final written decisions, 35 (64%) resulted in final written decisions in which all instituted claims were unpatentable and the remaining 20 (36%) resulted in final written decisions in which all instituted claims were not unpatentable. Of the duplicative final written decisions that were not joined, the PTAB found that in 10 (59%) all instituted claims were unpatentable and that in the remaining seven (41%) all instituted claims were not unpatentable (see figure 2). In other words, slightly more challenged drug patents lost claims when duplicative inter partes reviews were not joined, but the difference was minimal.
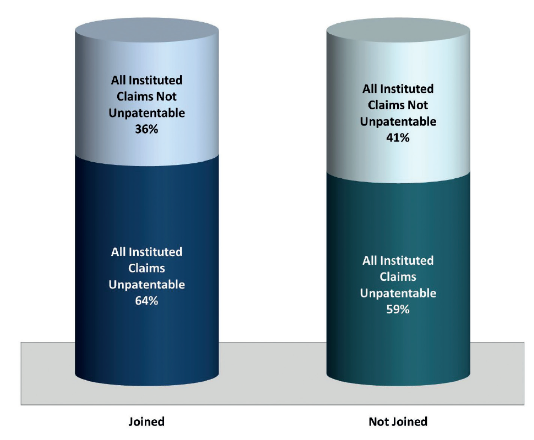
Figure 2: outcome of duplicative final written decisions
The biggest difference can be seen when the statistics for these duplicative final written decisions are compared with those for final written decisions issued on drug patents that have, to date, only been challenged in one inter partes review resulting in a final written decision ('single final written decisions'). The difference in these numbers is much more significant. In total, combining all duplicative final written decisions on drug patents as of July 20 2017 (joined and not joined), the PTAB found all instituted claims unpatentable in 45 (63%) and all instituted claims not unpatentable in 27 (38%) of these final written decisions.
Comparing this to the same metrics for single final written decisions on drug patents, in single final written decisions, the board found:
- all instituted claims unpatentable in 19 (43%);
- some instituted claims patentable and some unpatentable in two (5%); and
- all instituted claims not unpatentable in 23 (52%) final written decisions (see figure 3).
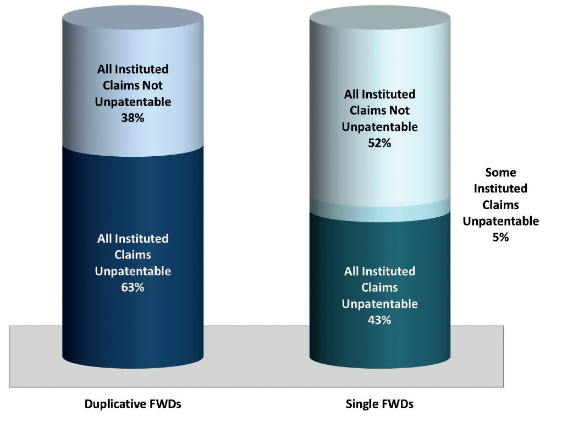
Figure 3: outcome of single final written decisions
In other words, challenged claims of drug patents are much more likely to be found unpatentable when there have been multiple rather than single final written decisions on the same patent – 63% versus 43% – although this may be attributed to the strength of the patents themselves as opposed to a second attempt being more successful. There are a number of observations that can be made and lessons that can be learned from these duplicative final written decisions. First, even outside joinder (where a party seeks to join a petition) – at least to date – the PTAB appears to be coordinating, and is issuing, similar final written decisions on these inter partes reviews, even when the oral hearings are held and decisions are issued months apart. Second, thus far, all of these duplicative final written decisions have assessed the patentability of instituted claims on the ground of obviousness, and all have assessed the patentability of instituted claims based on at least one prior art reference cited during prosecution. Third, despite criticism that the PTAB tends to give short shrift to secondary considerations, where a prima facie case of obviousness was found, it included in its analysis consideration of objective evidence of non-obviousness (including unexpected results, long-felt but unmet need, industry praise and commercial success). Where this evidence was deemed unsuccessful in tipping the balance in the patent owner's favour, the PTAB generally reasoned that this was because of insufficient evidence of a nexus between the objective evidence and the claimed invention. There has been at least one notable exception to this: the Prolensa final written decisions (IPR2015-00902 and -01099).
Prolensa final written decisions
In the Prolensa final written decisions, the PTAB was persuaded that on the preponderance of the evidence, objective evidence of unexpected results, long-felt need and commercial success presented by the patent owner outweighed the petitioners' prima facie case. The Prolensa inter partes reviews concerned challenges to all 30 claims of US patent 8,669,290 ('the '290 patent'), owned by Senju Pharmaceutical as obvious based on one prior art patent listed on the face of the '290 patent and one new prior art patent that was not cited during prosecution. The '290 patent concerns aqueous liquid preparations for ophthalmic administration, consisting of two components: bromfenac (or its salts or hydrates) (a non-steroidal anti-inflammatory drug (NSAID)) and tyloxapol (which stabilises the bromfenac component). The PTAB found that the prior art patent listed on the face of the '290 patent disclosed every limitation of claim 1 of the '290 patent, except that it disclosed use of polysorbate 80, instead of a specific concentration of tyloxapol. This limitation was satisfied by the new prior art patent, which disclosed an aqueous ophthalmic preparation of a different NSAID, diclofenac potassium salt, and tyloxapol, in an amount that fell within the claimed concentration range. Based on the record presented at the oral hearings held in April and June 2016, the PTAB found that a person of ordinary skill in the art would have known that polysorbate 80 and tyloxapol could be substituted successfully and predictably because these compounds had previously been used interchangeably in ophthalmic formulations. The PTAB also found that this known interchangeability was sufficient to establish a prima facie case of obviousness, even in the absence of an express suggestion to use tyloxapol. The PTAB then turned its attention to Senju's evidence of unexpected results, commercial success and industry praise. Based on the record, the PTAB found, among other things, that:
- substituting polysorbate 80 for tyloxapol – including, unexpectedly and counter-intuitively, a reduced concentration of tyloxapol – had the surprising and unexpected result of a significant improvement in the stability of bromfenac;
- the use of tyloxapol lowered the pH to close to that of natural tears and meant that amounts of other irritating ingredients could also be reduced;
- the claimed inventions were embodied in Prolensa, a product that is commercially successful at least in part because it lacked the burning and stinging side effects of other treatments; and
- Prolensa had received significant industry acclaim based on benefits flowing from the use of tyloxapol.
In short, the Prolensa inter partes reviews are a patent owner's success story – they show that strong, robust evidence of objective indicia of non-obviousness may be enough to overcome a prima facie case of obviousness, even in inter partes reviews and even in cases where the patent at issue has been challenged in subsequent inter partes review petitions.
At the other end of the spectrum are the recently issued Humira final written decisions (IPR2016-00172, 00408 and 00409). The Humira inter partes reviews concerned challenges to all five claims of US patent 8,889,135 ('the '135 patent'), owned by AbbVie Biotechnology. Petitioners Coherus Biosciences and various Boehringer Ingelheim companies challenged these claims as obvious based on various combinations of prior art references, all of which were cited during the prosecution of the '135 patent. The '135 patent claimed methods for treating rheumatoid arthritis by subcutaneously administering an anti-tumor necrosis factor α ('anti-TNFα') antibody – which has the six complementary determining regions and heavy chain constant region of D2E7 (a recombinant human anti-TNFα antibody) – in a specific dosage regimen (40mg once every 13-15 days). The PTAB found that, collectively, the cited prior art disclosed each and every element of all of claims 1-5 and there was a motivation to combine the various teachings with a reasonable expectation of success. As to objective evidence of non-obviousness, AbbVie argued unexpected results, long-felt unmet need and commercial success. However, the PTAB was not persuaded – it found that:
- there was insufficient evidence that the efficacy of the claimed invention would have been unexpected;
- long-felt need may instead have been satisfied by the introduction of the first fully human anti-TNFα antibody; and
- while the commercial embodiment of the claimed inventions, Humira, was commercially successful, it was not clear from the evidence presented whether there was a nexus to the claimed inventions or whether, for example, commercial success was a result of the formulation, or the result of known and patented fully human D2E7 antibody.
In three final written decisions issued in May and July 2017, the PTAB found all five challenged claims unpatentable.(2)
While multiple inter partes reviews challenging claims of the same drug patent increase the risk that claims will be found unpatentable, the PTAB appears to be coordinating these duplicative inter partes reviews, even outside joinder. Also, patent owners should not lose hope as to secondary considerations – the Prolensa inter partes reviews are a testament to the fact that robust objective evidence of non-obviousness can prevail. In any event, patent owners must take care to take consistent positions across their patent portfolios when responding to multiple challenges.
This article was first published by the International Law Office, a premium online legal update service for major companies and law firms worldwide. Register for a free subscription.
For further information on this topic please contact Corinne E Atton or Ha Kung Wong at Fitzpatrick, Cella, Harper & Scinto by telephone (+1 212 218 2100) or email ([email protected] or [email protected]). The Fitzpatrick, Cella, Harper & Scinto biologics group website can be accessed at www.BiologicsHQ.com.
Endnotes
(1) The number of individual final written decisions issued was 24 because each decision ruled on at least two joined inter partes review proceedings.
(2) Patent owner AbbVie filed notices of appeal to the Federal Circuit concerning IPR2016-00172 on July 14 2017 (Fed Cir 17-2304), and concerning IPR2016-00408 and -00496 on July 31 2017 (Fed Cir 17-2362 and 17-2363).
An earlier version of this update appeared in Intellectual Property Magazine, September 2017.




