The Consolidated Appropriations Act, which was enacted on 27 December 2020, requires drug manufacturers and licence holders to market biologic drugs and disclose all patents that cover their products to the Food and Drug Administration (FDA).
By increasing transparency, the act aims to force manufacturers to conform to rules which have proven successful in promoting the development and use of small-molecule generic drugs.
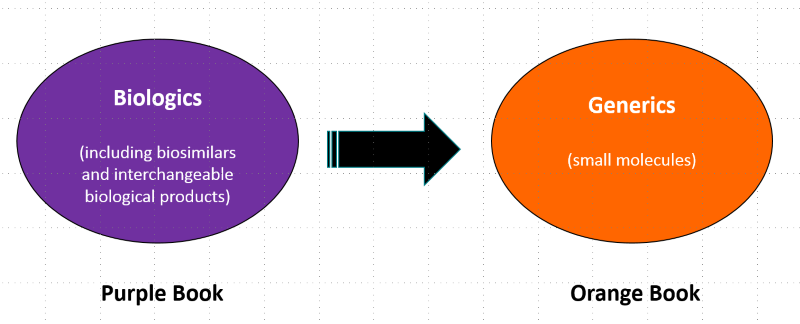
Thus, in addition to the list of Approved Drug Products with Therapeutic Equivalence Evaluations (the 'Orange Book'), which lists approved small-molecule generic drugs, the FDA will now maintain a Database of Licensed Biological Products (the 'Purple Book'), which will list approved biosimilar and interchangeable biological products.
In 2009 Congress enacted the Biologics Price Competition & Innovation Act (BPCIA) to provide an abbreviated pathway for biosimilars to gain FDA approval through the submission of an abbreviated biologics licence application. The goal was to reduce the price for biologics, as the Hatch-Waxman Act 1984 did for small-molecule drugs.
The BPCIA includes two main statutory provisions:
- 42 US Code (USC) Section 262(k) – which relates to the regulatory aspects of the BCPIA; and
- 42 USC Section 262(I) – which outlines a framework for applicants and reference product sponsors to resolve patent disputes (the so-called 'patent dance').
Within 180 days, the FDA will provide more information to the public in the Purple Book, including:
- a list of each biological product (including 'deemed' biologics under the BPCIA's transition provision), by non-proprietary name, for which a biologics licence is in effect;
- the date of licensure and the application number;
- the licensure status and, as available, the marketing status; and
- exclusivity periods.
Reference product sponsors must provide patent information to the FDA within 30 days of the date on which the information is provided to the biosimilar applicant as part of the 'patent dance' for biosimilar approval.
The FDA will include this patent information when it revises the Purple Book every 30 days.
The FDA will publish any subsequent or supplemental list of patents provided to a biosimilar applicant.
There will be a request for public comment in 2023 regarding what should be added or deleted from the list.





