Switzerland is home to a globally unique life sciences cluster. Some of the world's largest pharmaceutical and chemical companies have their roots in Switzerland and many more have chosen to establish key research and production sites there. The close cooperation between research and development forms the basis for the success of more than 300 resident biotech companies. And in no other country does medical technology contribute so much to gross domestic product as in Switzerland. In a country where 33% of export goods are chemical-pharmaceutical products, it is not surprising that patents play an important role. Switzerland is the European country with the most patent filings relative to population size and, unsurprisingly, most Swiss patent applications come from the life sciences field.
A strong patent system also needs strong enforcement means. This article provides a brief overview of the Swiss patent litigation system and highlights some trends in life sciences patent litigation.
Since 2012, a single federal court has heard all lawsuits relating to patent infringement and validity: the Federal Patent Court, located in St Gallen, one hour east of Zurich. The Federal Patent Court is composed of both legally and technically trained judges. Most judges are part-time judges who also practise as patent attorneys or attorneys at law. These judges have in-depth expertise in most technical fields, which allows composing the adjudicating panel to fit the necessities of each individual case.
The Federal Patent Court has exclusive jurisdiction over actions regarding the validity and infringement of patents, compulsory licences and applications for preliminary measures. Unlike that of Germany, Switzerland's patent litigation system is not bifurcated (ie, the Federal Patent Court can hear both validity and infringement issues in the same proceedings). For patent licensing disputes, the Federal Patent Court has concurrent jurisdiction with the cantonal courts.
Considering the strong role of the life sciences industry in Switzerland, it is unsurprising that most cases heard by the Federal Patent Court concern the life sciences sector (see Figure 1).
Switzerland is a multilingual country. In proceedings before the Federal Patent Court, the parties can submit their briefs and pleadings in any of the Swiss official languages (German, French, Italian and Romansh) and – if both parties agree – in English. Most proceedings in the life sciences field are in English, which is advantageous for international companies as it allows smooth coordination in multinational disputes and saves translation costs and time.
Main proceedings at the Federal Patent Court last approximately 18 to 24 months from commencement to a first-instance judgment. This time comes down to four to 10 months for preliminary injunction proceedings. Ex parte injunctions are generally rare and may be granted only in exceptional cases in life sciences matters where the issues at stake tend to be complex.
Looking at the completion of patent litigation proceedings in Switzerland, the high settlement rate is striking. Almost 60% of all cases end with a settlement; although this figure is not representative of life sciences disputes with multinational angles, which are rarely settled in Swiss courts. This particularity is due to the structure of proceedings, more precisely the so-called 'preparatory hearing' or 'settlement hearing', which is held after the first exchange of briefs and where a court delegation typically consisting of a legal and a technical judge will give its preliminary opinions on how it would decide the case based on the arguments and evidence submitted so far. This preliminary assessment serves the purpose of encouraging settlement discussions between the parties without prejudicing the outcome of the case. Judgments of the Federal Patent Court can be appealed to the Federal Supreme Court, whose review is limited to questions of law. Appeal proceedings last approximately six to nine months. Monetary claims (eg, damages) or surrender of profits claims are typically dealt with at a second stage of the proceedings. This means that infringement (and validity) is assessed first and, if confirmed, the defendant will be ordered to provide information on its sales and profits. Thereafter, the plaintiff will need to substantiate its monetary claims and the court will decide on the quantum. However, most cases are settled after a finding of infringement.
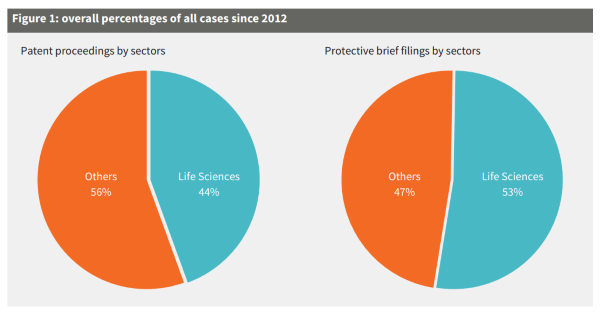 Figure 1
Figure 1
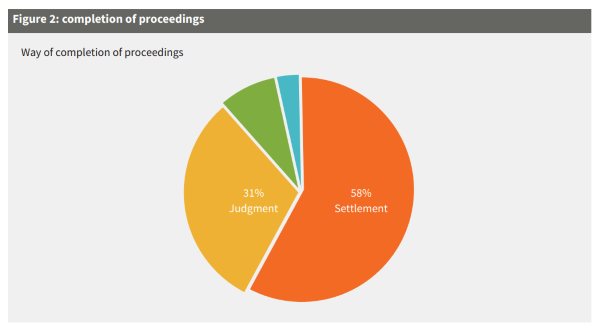 Figure 2
Figure 2
Finally, a word about discovery means in Swiss proceedings: US-style pre-trial discovery is not available in Switzerland. Importantly, the parties are not generally obliged to disclose relevant documents and materials to their opponent. However, some limited yet effective options are available for obtaining evidence before initiating infringement proceedings. For instance, the Federal Patent Court can order, as a preliminary measure, a description or seizure of the allegedly infringing product, process and means of production based on a prima facie showing of infringement. The obtained findings can then be used in later infringement proceedings in Switzerland and abroad.
Overall, Switzerland offers an attractive and competitive forum for patent litigation in general and particularly in the life sciences field. The Federal Patent Court has established an excellent reputation internationally. The fact that the court can rely largely on its own technical expertise without the need to appoint external experts allows for dealing with complex actions within reasonable times and at comparatively low costs.
Latest developments regarding SPCs
Supplementary protection certificates (SPCs) have been hotly debated at the Federal Patent Court and the Federal Supreme Court in recent years, resulting in important judgments for the life sciences industry.
Catalogue of nullity grounds in Patent Act is exhaustive
The Federal Patent Court and the Federal Supreme Court recently decided on the question of whether SPCs can be invalidated based only on the nullity grounds explicitly listed in Article 140k of the Patents Act or if additional grounds (eg, a wrong reinstatement into the six months application deadline for SPCs) can also cause the invalidity of an SPC. A generic manufacturer had invoked nullity of an SPC as a result of an allegedly wrong reinstatement by arguing with reference to several judgments of the European Court of Justice (ECJ), the German Federal Patent Court and the High Court of England and Wales that EU law does not restrict the catalogue of nullity grounds.
Both Swiss courts found that the relevant provision (Article 140k of the Swiss Patent Act, which basically corresponds to Article 15 in connection with Article 3 of the EU SPC Regulation (Regulation 469/2009/EC)) lists in an exhaustive manner all nullity grounds that can be invoked against SPCs (FSCJ 145 III 91; FPCJ O2017_016). The six-month application deadline for an SPC is not listed in this catalogue and therefore an allegedly wrong reinstatement of the deadline cannot be invoked as a nullity ground in civil proceedings. In deciding this, the Swiss courts also looked at European law and case law and expressed the view that there is no basis to assume that the catalogue of nullity grounds of Article 15 in connection with Article 3 of the EU SPC Regulation is not intended to be exhaustive or has been enlarged by judgments of the ECJ or national courts. This consideration is interesting because the question at issue is discussed controversially by some European scholars.
New test for combination products: disclosure theory replaces infringement test
Gilead's Truvada has been the subject of interesting litigations around the world and Switzerland is no exception. In Switzerland, the key question was the applicable test to analyse infringement of an SPC. The infringement test had been traditionally applied since the Federal Supreme Court's Fosinopril decision in 1998 (FSCJ 124 III 375). Consequently, it was not necessary that the product of an SPC be named and described in the basic patent as long as it was covered by the scope of the patent. Notably, at the time of the Fosinopril judgment, the infringement test was also the pertinent test in the European Union and the Federal Supreme Court had explicitly stated that EU practice must be taken into account in view of the large harmonisation of the Swiss SPC rules with EU regulation. The infringement test had been applied ever since, although newer judgments somewhat reflected the considerations of the ECJ that had meanwhile moved to the disclosure theory (eg, the Federal Administrative Court had considered the 'patented idea of invention' and the 'core of the invention' in its Panitumumab decision in 2011).
In the Truvada case the Federal Supreme Court reversed this case law in its 2018 landmark decision (FSCJ 144 III 285). The aim to harmonise the level of protection granted by Swiss SPCs with the level of protection applicable in the European Union also brought a certain necessity to follow EU practice in this respect, at least to the extent that it reflects the solution enacted by the Swiss legislature and as long as there are no better reasons for a deviant Swiss practice. The Federal Supreme Court concluded that the ECJ's disclosure theory, which was introduced with the Medeva judgment and has been further developed since, should now also be applied in Switzerland; however, for new SPCs only. SPCs that have been granted before the 11 June 2018 judgment remain subject to the infringement test, whereas newer SPCs, including all pending applications, are subject to the disclosure test.
Lack of legal certainty regarding second medical use claims
Surprisingly, infringement of second medical use claims has not been the subject of any court decisions in Switzerland so far and there is therefore a degree of legal uncertainty in this respect.
Some Swiss scholars suggest relying on the German doctrine of 'manifest preparation'. According to this doctrine, the substance recited in the claims must be manifestly prepared for use in the treatment of the protected indication to constitute an infringement of a Swiss-type claim. Traditionally, the focus was on the packaging of the allegedly infringing product and the instructions for its use to identify the indications that the product is intended to treat. This approach was echoed by the European Patent Office Technical Board of Appeal but has also been criticised by some scholars as being overly narrow. More recently, the German courts have taken a broader view of the scope of protection of Swiss-type claims by focusing on the suitability of the product for the patented use rather than its external presentation. In the European-wide patent litigation Warner-Lambert v Generics, the UK Supreme Court adopted yet another approach – a form of subjective intention test, which permits any means of proof to identify the indications for whose treatment the product is intended. It remains to be seen what approach the Swiss courts will take regarding the test for infringement of second medical use claims. In any event, the question of how infringement of second medical use claims will be dealt with by the Swiss courts will certainly remain a hot topic in Swiss life sciences litigation for some years, as the uncertainties have been accentuated by a recent change in Swiss patent law.
New exceptions pose new challenges in originator and generics battles
Until 2019, Swiss patent law had no special rules exempting medical professionals from patent infringement. The Patent Act set out that "methods for treatment by surgery or therapy and diagnostic methods practised on the human or animal body" do not constitute patentable subject matter (Article 2(2)(a) of the Patent Act; see also Article 54(4) and (5) of the European Patent Convention).
In 2011 the Federal Supreme Court handed down a decision regarding the dosage regimen of alendronate (FSCJ 137 III 170). The Federal Supreme Court confirmed that dosage regimen claims are fundamentally patentable. However, the it indicated that such claims may result in medical practitioners being sued for patent infringement and suggested that the legislature consider whether there is need for appropriate action.
In a quick reaction and without wide public consultation, the Federal Parliament passed a bill amending the Patent Act. The new provision entered into force in 2019. The new law sets out that the effects of the patent do not extend to:
actions in the context of a medical activity which relates to an individual person or animal and concerns medicinal products, in particular the prescription, supply or use of medicinal products by persons legally entitled to do so.
Because of a peculiarity of Swiss patent law, one consequence of these new provisions seems to put owners of second medical use patents into a more difficult situation than before. Swiss patent law has no specific doctrine of indirect infringement. Contributory infringement (including inducement, assistance or facilitation of infringement) is considered to be accessory to direct infringement; it requires the existence of a direct infringement that is objectively unlawful and that takes place on Swiss territory. Since the activities of prescribing physicians or pharmacists are now explicitly excluded from the scope of a patent, there does not seem to be an objectively unlawful act of direct infringement, given that the prescription or sale of a drug for a patent-protected indication is now outside the scope of the relevant patent. It is therefore questionable under what legal theory manufacturers, importers or distributors of generic drugs can be made liable for the infringement of second medical use claims.
Swiss legal doctrine has not yet solved this conundrum and there is still no case law on this new provision. Nevertheless, Swiss scholars agree that the legislature did not intend to free generics manufacturers, importers or distributors from infringement lawsuits based on second medical use claims. Such an outcome would not be compliant with Switzerland's obligations under Article 30 of the Agreement on Trade-Related Aspects of Intellectual Property Rights. It is therefore expected that the Swiss courts will find creative ways around this issue, by applying by analogy general Swiss tort law principles or by interpreting the existing theory of 'manifest preparation' of a product for a patented use more broadly than in the past.
How to bring public health considerations into the battlefield
In recent times, injunction requests in the life sciences sector have increasingly triggered the question of the potential impact that such measures could have on patients. In particular, when preliminary injunctions are at stake, the question arises as to whether the court should or even must be able to take public health considerations into account (eg, the patients' interest in having access to the most effective treatment).
When looking at common law jurisdictions such as England and Wales, the consideration of public health interests in injunction proceedings seems to be an established practice. The English courts consider injunctive relief as an equitable remedy that is subject to the court's broad discretion. They accept objections against the granting of a preliminary injunction based on public health interests and consider them under Article 3 of the European Enforcement Directive, which states that measures to enforce intellectual property should be "fair and equitable" and "effective, proportionate and dissuasive". In contrast, in Switzerland the default rule in patent law is that a finding of infringement generally entitles the owner to an automatic injunction. Hence, the statutory rules on remedies leave no room for discretionary decision making or for a weighing of interests. Under Swiss law, a court will grant a preliminary injunction if it is prima facie satisfied that:
- the respondent infringes or is about to infringe the applicant's valid patent rights;
- the respondent's patent infringing acts threaten to cause harm to the applicant that is not easily rectified;
- the grant of the preliminary injunction is urgent; and
- the requested preliminary injunction is proportionate.
Under the requirement of proportionality, the court will ask whether the requested injunction is suitable and necessary for the infringing acts to cease and weigh the parties' interests for and against the requested injunction. In a recent judgment, the Federal Patent Court made it clear that the Swiss legislation leaves no room for the consideration of public interests as a part of the proportionality assessment. It held that under Swiss law, the protection of public interests is secured by the Patent Act through a conclusive system of compulsory licences (Federal Patent Court Judgment O2019_002, 15 August 2019).
In particular, Article 40 of the Patent Act allows the court to grant a party a compulsory licence in the public interest under the condition that the applicant previously attempted to receive a regular licence from the rights holder by submitting a licence offer to the patentee at reasonable market conditions. So far, there have been no cases in Switzerland where this provision was applied and a compulsory licence in the public interest was granted. Accordingly, it remains equally unclear whether a party could request a compulsory licence at the stage of preliminary injunction proceedings (which was affirmed in Germany in BGH X ZB 2/17 – Raltegravir). However, a case is pending with the Federal Patent Court which may soon shed light on the requirements for the grant of a compulsory licence in the public interest.
In Switzerland public health considerations cannot be invoked to prevent an injunction in patent infringement proceedings. However, such considerations may justify a compulsory licence, which must be explicitly claimed under a showing of the respective requirements.
Life sciences cases occupy a prominent place in Swiss patent litigation. Thanks to a relatively new court with specialised judges, the quality and speed of proceedings rival those of big European patent jurisdictions. The unique possibility (on the European continent) of conducting fully fledged litigation in English (including briefs and hearings) reduces both the cost and complexity for foreign litigants.
Thanks to Switzerland's leading position in the life sciences industry, life sciences litigation is an active area. However, many legal questions remain unresolved, resulting in uncertainty but opening up a degree of flexibility. Recently, certain groundbreaking decisions of the Swiss courts disrupted the law regarding SPCs, fundamental statutory amendments may have rewritten the law of second medical use patents and ongoing disputes may further reshape the way that public interest issues are taken into account in civil proceedings.
An earlier version of this article was published in IAM's Life Sciences: Key issues for senior life sciences executives.





